
Reduce fat. Build muscle.1,2
Reveal their true potential with a personalized approach to body sculpting. truBody® optimizes results by combining two proven technologies to take your patients beyond their best.
If it advances aesthetics and dermatology treatments, we’re working on it at Cutera®.
Every energy device company says they’re innovative. Only a few become truly transformative. With every breakthrough, we redefine the results that drive patient demand. We’ve redefined acne treatment forever. We pushed body sculpting customization beyond the limits. Imagine what we’ll do next.
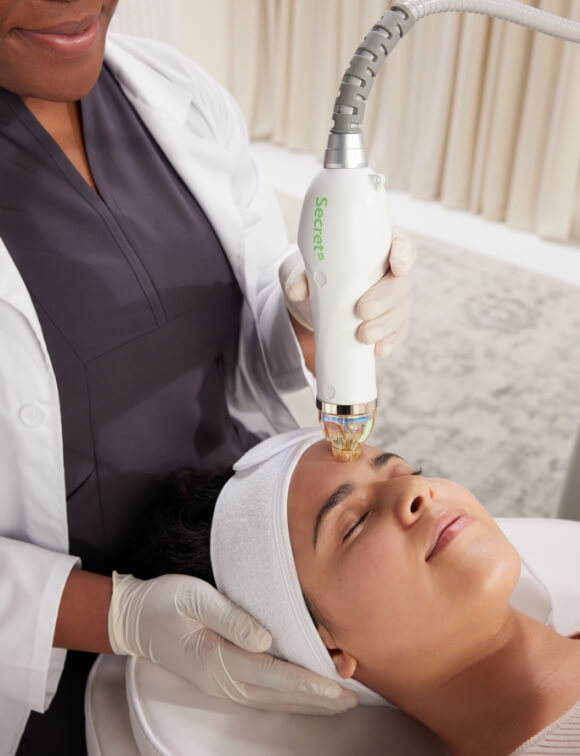


More mindful science creates more impactful products. We are developers of brighter solutions. We are creators of confidence. And there’s a science behind it all.

We invest in clinical research and development so you have the data and evidence you need to confidently share the visible results your patients desire.







1. truSculpt flex Operator Manual. Cutera, Inc. D0002529, Rev. A, 10/19. 2. truSculpt iD Operator Manual. Cutera, Inc. D2023, Rev. A, 08/18.